

WELCOME TO THE PATHOBIOLOGY LEARNING THEATRE
Immerse yourself in IL-17A and IL-17F and their role in the inhibition of inflammation
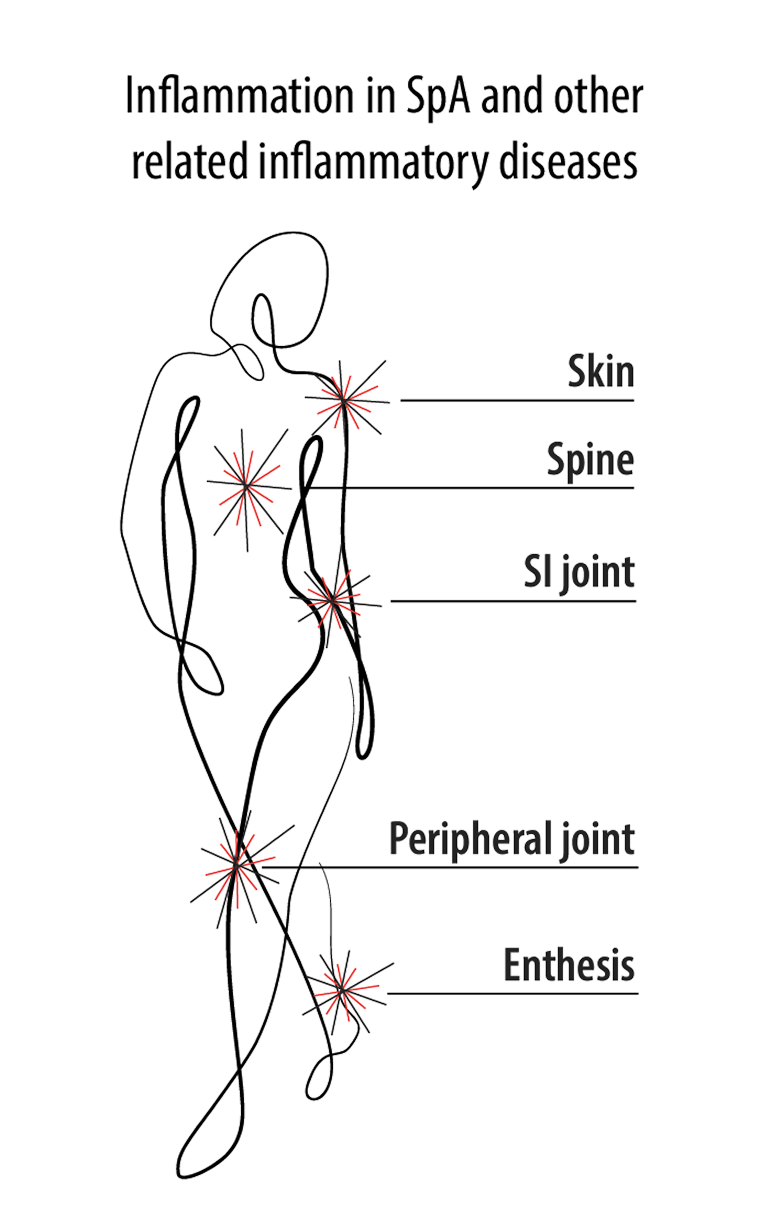
The chronic inflammatory pathogenesis of SpA is complex, is multifactorial, and arises from external factors1-3
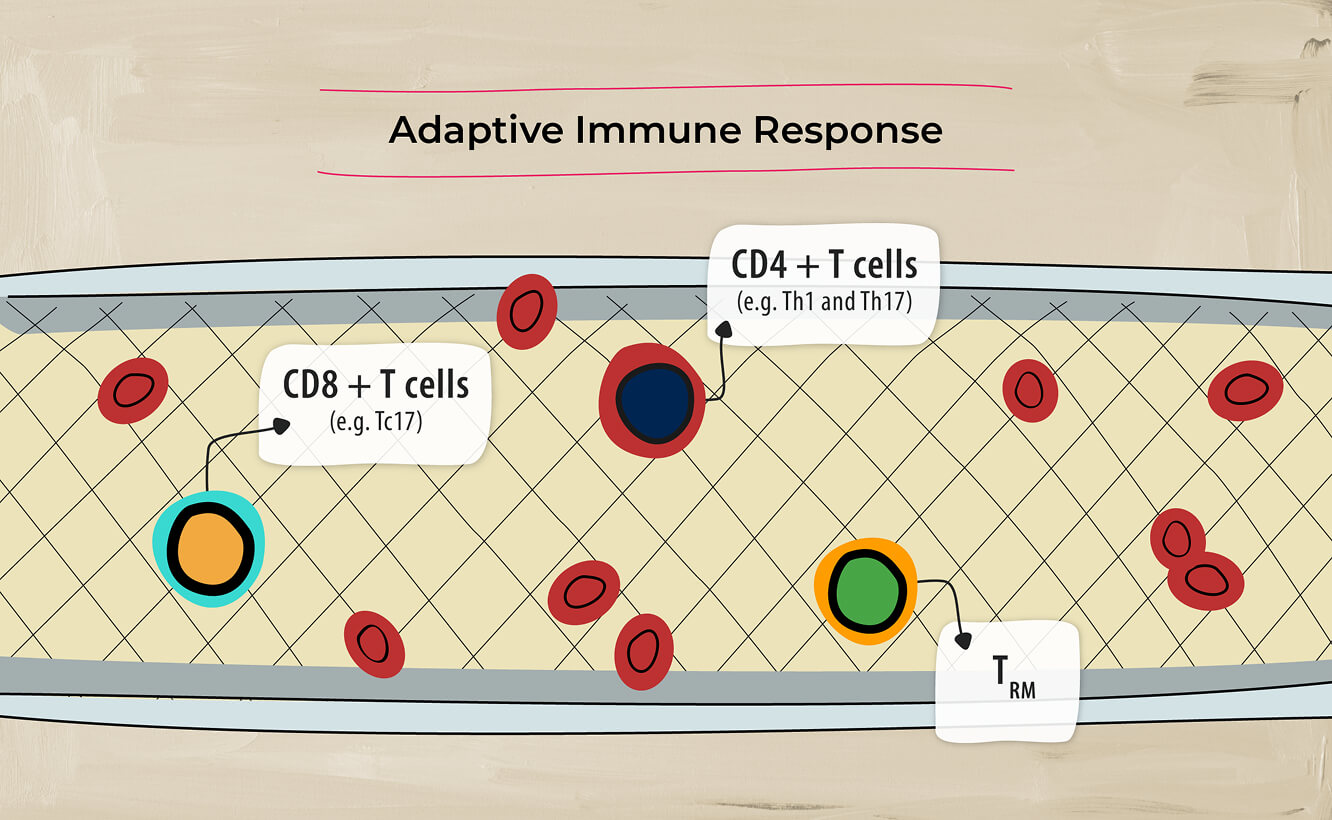
The inflammatory pathways associated with axSpA involve both adaptive immune response4…
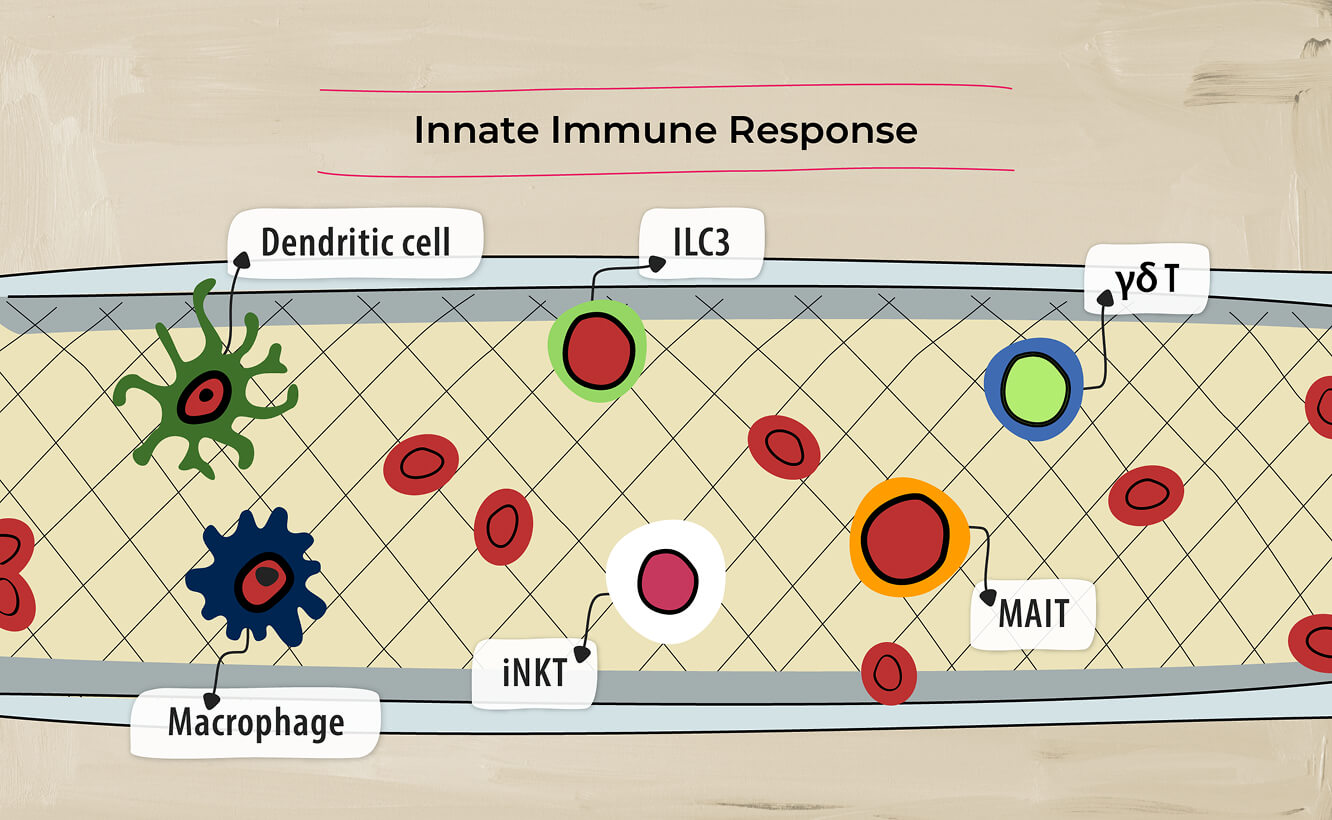
and innate immune response.4
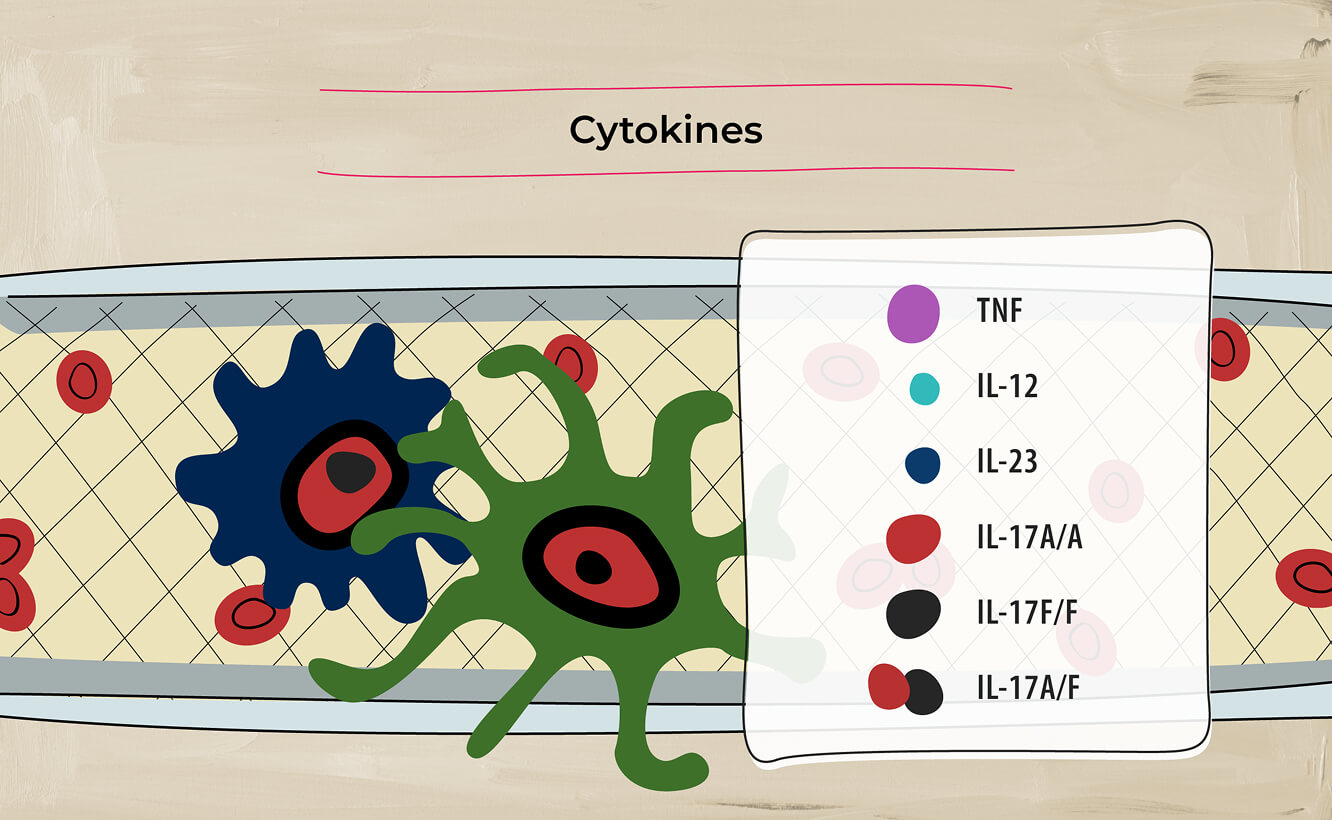
Pro-inflammatory cytokines have established roles in driving inflammation in axSpA, including TNF, IL-12, IL-23, IL-17A and IL-17F.3
Uncover the mechanisms driving SpA diseases, including the role of IL-17A, IL-17F, TNF, and IL-23
A closer look at the IL-17 family of cytokines
The IL-17 family of cytokines includes IL-17 A, B, C, D, E, and F. These cytokines play diverse roles in the pathogenesis of various inflammatory diseases and homeostasis.5-8 Watch videos by Prof. Iain McInnes on the role of cytokines in axSpA
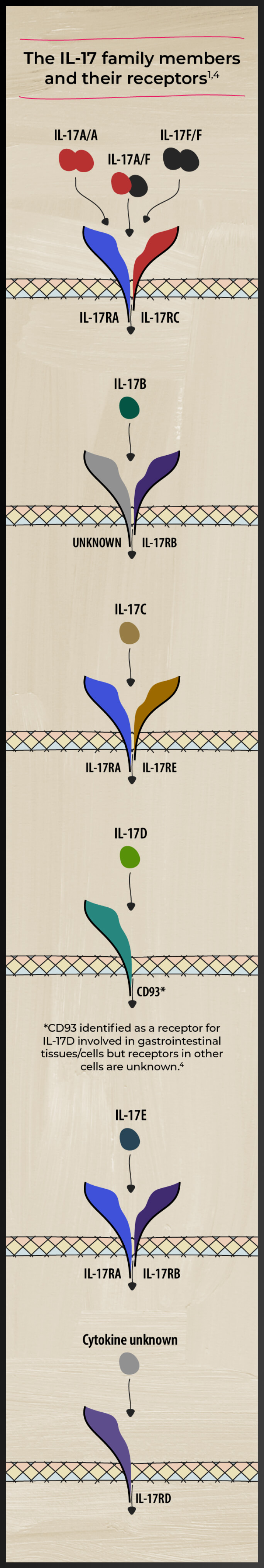
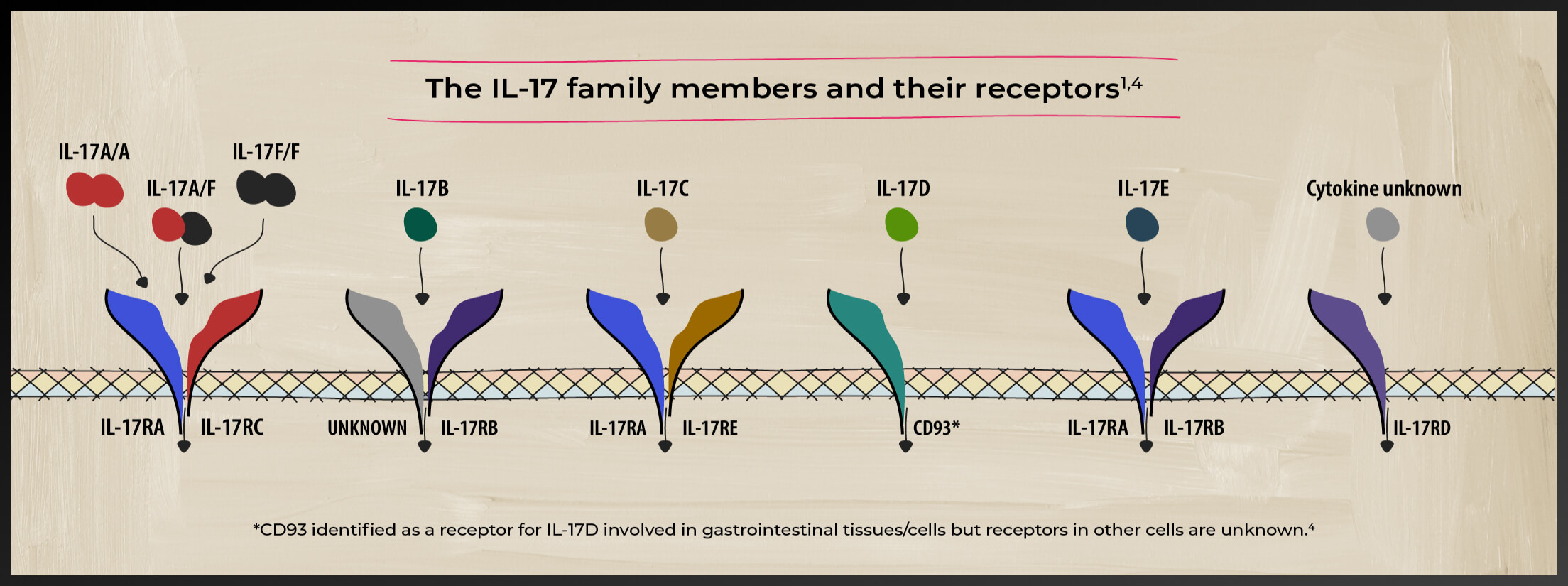
Studies have identified both IL-17A and IL-17F as players in axSpA-related inflammation5
IL-17A and IL-17F share similarities
IL-17A and IL-17F share ~50% structural homology and have a similar pro-inflammatory function, signaling via the same receptor complex.11
Together IL-17A and IL-17F synergize with other cytokines, including TNF, IL-1β, and IL-22, to amplify the inflammatory response. This causes prolonged inflammation in sites like the axial skeleton11-14
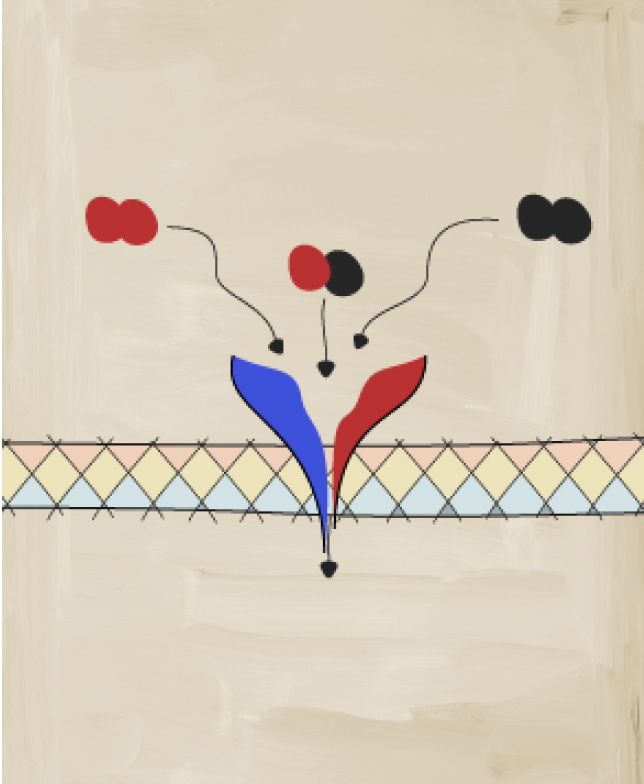
Receptor Complex
Studies have shown that IL-17A and IL-17F cooperate with other mediators of inflammation11
The importance of IL-17A and IL-17F in bone pathogenesis
The contribution of IL-17A and IL-17F to pathological bone formation has been shown in a human periosteum-derived stem cell model of osteogenic differentiation, indicating the importance of IL-17A and IL-17F in bone pathogenesis15
The role of the IL-23–IL-17 axis
IL-23 contributes to the lineage maintenance and proliferation of Th17, including the production of IL-17A and IL-17F from Th17 cells. This process is commonly referred to as the IL-23–IL-17 axis, and has been implicated in several inflammatory diseases, including axSpA.16-19
Watch a video with Prof. Iain McInnes as he discusses the IL-23–IL-17 axis in axSpA
Cells of the innate immune system can also produce IL-17A and IL-17F independently of IL-23.14, 20-22
IL-17A and IL-17F independently cooperate with TNF to drive inflammation by amplifying the production of IL-6 and IL-8 in synoviocytes.14,21
Incomplete blockade of IL-17A may result in residual disease activity6,26
Several effective biologic and small-molecular drugs targeting specific pro-inflammatory cytokines and signaling pathways have been developed, including inhibitors of tumor necrosis factor-α (TNF-α), interleukin (IL)-23, IL-12/23, and IL-17.26.27
Across domains, the number of patients who achieve remission with single cytokine approaches is limited. There’s a need for treatments that offer long-term and sustained efficacy.27
Even when treated, many patients may not have a sufficient response to treatment or only achieve partial remission. In addition, for some patients, treatments may lose their effectiveness over time.26
Focusing on the role of both IL-17A and IL-17F
While IL-17A and IL-17F are similar in many ways, their roles may differ28
These differences may be due to differences in expression ratios across various sites of the body, differential regulation, and differences in potency.28 For example, although IL-17F is found at higher levels (up to 30-fold) in lesional skin and serum of patients with PSO, IL-17A has a more potent pro-inflammatory effect.27
IL-17A acts in early inflammation, and IL-17F takes center stage later in the process27
Regulation of IL-17A and IL-17F expression varies across time. IL-17A is quickly generated following the stimulation of Th17 cells, whereas the expression of IL-17F rises gradually, reaching greater levels at later stages of activation.27
On the other hand, unlike IL-17F, IL-17A expression is not maintained by ongoing T cell activation. This may imply that whereas IL-17A is significant at the beginning of inflammation, IL-17F would become more significant as the inflammation progresses.27
Dig deeper into the dual inhibition of IL-17A and IL-17F
Select a hotspot below to reveal more information
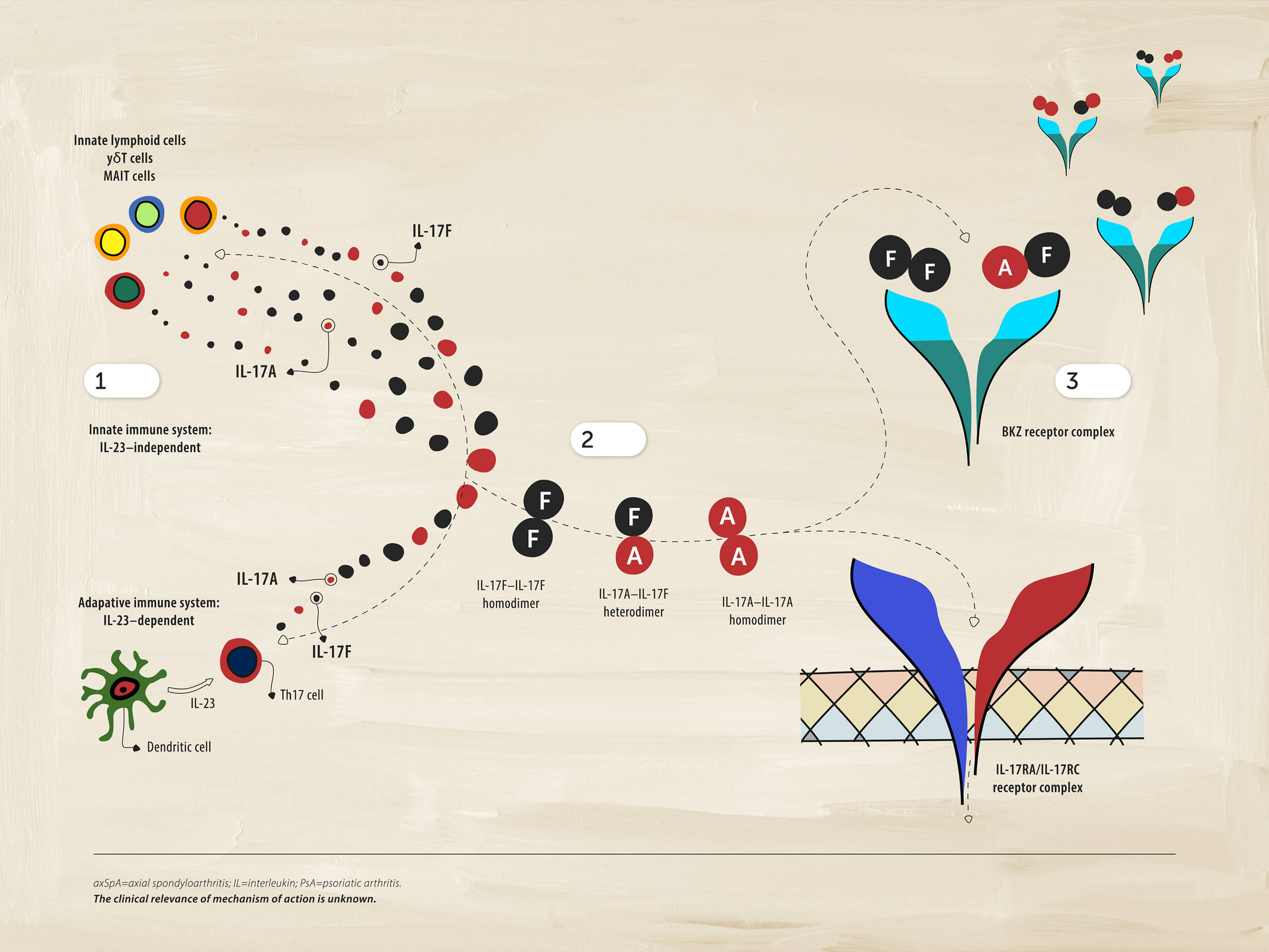
1 Immune cells
Cells of the immune system can also produce IL-17A and IL-17F independently of IL-23. This aspect of the pathobiology of axSpA may be distinct from PsA, where the IL-23-IL17 axis still appears to play an integral role in the disease.
2Driving inflammation
IL-17A and IL-17F form pairs of homodimers and heterodimers that bind to the same receptor and drive inflammation.
3Dual inhibition
Dual inhibition of IL-17A and IL-17F provides suppression of inflammation.


Learn more about key cytokines that could be dysregulated, leading to inflammation


NEXT ROOM IN THE PATHOBIOLOGY EXHIBIT
Immune dysregulation
Explore how cytokine dysregulation drives inflammation in axSpA.
PREVIOUS ROOM
Overview and role of cytokines
Become a member of the RheuMuseum and be the first to know about new exhibits
- Furst DE, Louie JS. Targeting inflammatory pathways in axial spondyloarthritis. Arthritis Res Ther. 2019;21(1):135. Published 2019. doi:10.1186/s13075-019-1885-z
- Watad A, Bridgewood C, Russell T, et al. The early phases of ankylosing spondylitis: emerging insights from clinical and basic science. Front Immunol. 2018;9:2668. doi: 10.3389/fimmu.2018.02668
- Rezaiemanesh A, Abdolmaleki M, Abdolmohammadi K, et al. Immune cells involved in the pathogenesis of ankylosing spondylitis. Biomed Pharmacother. 2018;100:198-204. doi:10.1016/j.biopha.2018.01.108
- McGonagle DG, Mcinnes IB, Kirkham BW, et al. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies [published correction appears in Ann Rheum Dis. 2020;79(1):e12]. Ann Rheum Dis. 2019;78(9):1167-1178. doi:10.1136/annrheumdis-2019-215356
- Yeremenko N. Out of the shadow of interleukin-17A: the role of interleukin-17F and other interleukin-17 family cytokines in spondyloarthritis. Curr Opin Reumatol. 2021;33(4):333-340. doi:10.1097/BOR.0000000000000805
- Liu X, Sun S, Liu D. IL-17D: A Less Studied Cytokine of IL-17 Family. Int Arch Allergy Immunol. 2020;181:618-623. doi: 10.1159/000508255
- Brembilla NC, Senra L, Boehncke WH. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front Immunol. 2018;9:1682. doi: 10.3389/fimmu.2018.01682
- Goepfert A, Lehmann S, Blank J, et al. Structural analysis reveals that the cytokine IL-17F forms a homodimeric complex with receptor IL-17RC to drive IL-17RA-independent signaling. Immunity. 2020;52(3):499-512.5. doi:10.1016/j.immuni.2020.02.004
- Goepfert A, Lehmann S, Wirth E, Rondeau JM. The human IL-17A/F heterodimer: a two-faced cytokine with unique receptor recognition properties. Sci Rep. 2017;7 (1):8906. doi:10.1038/41598-017-08360-9
- Chung SH, Ye XQ, Iwakura Y. Interleukin-17 family members in health and disease. Int Immunol. 2021;33(12):723-729. doi:10.1093/intimm/dxab075
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77(4):523-532. doi:10.1136/annrheumdis-2017-212127
- Rosine N, Miceli-Richard C. Innate cells: the alternative source of IL-17 in axial and peripheral spondyloarthritis?. Front Immunol. 2021;11:553742. Published 2021. doi:10.3389/fimmu.2020.553742
- Taams LS, Steel KJA, Srenathan U, et al. IL-17 in the immunopathogenesis of spondyloarthritis. Nat Rev Rheum. 2018;14(8):453–466. Published 2018. doi: 10.1038/s41584-018-0044-2
- Noack M, Beringer A, Miossec P. Additive or synergistic interactions between IL-17A or IL-17F and TNF or IL-1β depend on the cell type. Front Immunol. 2019;10:1726. Published 2019. doi:10.3389/fimmu.2019.01726
- Shah M, Maroof A, Gikas P, et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open. 2020;6(2):e001306. doi:10.1136/rmdopen-2020-001306
- Tsukazaki H, Kaito T. The role of the IL-23/IL-17 pathway in the pathogenesis of spondyloarthritis. Int J Mol Sci. 2020;21(17):6401. Published 2020. doi:10.3390/ijms21176401
- Bridgewood C, Sharif K, Sherlock J, et al. Interleukin-23 pathway at the enthesis: The emerging story of enthesitis in spondyloarthropathy. Immunol Rev. 2020;294(1):27-47. doi:10.1111/imr.12840
- McGonagle D, Watad A, Sharif K, et al. Why inhibition of IL-23 lacked efficacy in ankylosing spondylitis. Front Immunol. 2021;12:614255. doi:10.3389/fimmu.2021.614255
- Mease P, Van den Bosch F. IL-23 and axial disease: do they come together? Rheumatology (Oxford). 2021;60(Suppl 4):iv28-iv33. doi:10.1093/rheumatology/keab617
- Wang R, Maksymowych WP. Targeting the interleukin-23/interleukin-17 inflammatory pathway: successes and failures in the treatment of axial spondyloarthritis. Front Immunol. 2021;12:715510. doi:10.3389/fimmu.2021.715510
- Sánchez-Rodríguez G, Puig L. Pathogenic role of IL-17 and therapeutic targeting of IL-17F in psoriatic arthritis and spondyloarthropathies. Int J Mol Sci. 2023;24(12):10305. doi:10.3390/ijms241210305
- Croes M, Öner FC, van Neerven D, et al. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone. 2016;84:262-270. doi:10.1016/j.bone.2016.01.010
- Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19(1):41-52. doi:10.1016/j.cytogfr.2007.10.004
- Cole S, Murray J, Simpson C, et al. Interleukin (IL)-12 and IL-18 synergize to promote MAIT Cell IL-17A and IL-17F production independently of IL-23 signaling. Front Immunol. 2020;11:585134. Published 2020. doi:10.3389/fimmu.2020.585134
- Russell T, Watad A, Bridgewood C, et al. IL-17A and TNF modulate normal human spinal entheseal bone and soft tissue mesenchymal stem cell osteogenesis, adipogenesis, and stromal function. Cells. 2021;10(2):341. Published 2021. doi:10.3390/cells10020341
- Deodhar A, Machado P, Morup M, et. al. Comparative efficacy and safety of bimekizumab in axial spondyloarthritis: a systematic literature review and network meta-analysis. Rheumatology. 2024;63:1195–1205. Doi.org/10.1093/rheumatology/kead598
- Tanaka Y, Shaw S. Bimekizumab for the treatment of psoriatic arthritis. Expert Review of Clinical Immunology. 2024;12(2):155-168
- Iwakura, Y. et.al. Functional specialization of interleukin-17 family members. Immunity Review. 2011;34(2);149-162